Quick Safety Issue 65: Managing packaged sterile supplies and devices
Issue:
Managing commercially prepared sterile supplies and devices can be challenging for healthcare organizations. In order to protect patients from infection and other potential harm from expired or compromised supplies and devices, organizations must identify the best location to store the supplies so that staff can readily access them, ensure the supplies are being stocked to the most optimal par levels, and that items have not passed their expiration dates. Additionally, the storage area must be maintained so that supplies are stored safely, in a manner where they are kept in good condition while protecting them from contamination.
The purpose of this Quick Safety is to provide guidance for managing and storing packaged sterile supplies within your facility with the goal to keep patients safe from infection and other potential harm from expired or compromised supplies and devices.
Device labeling
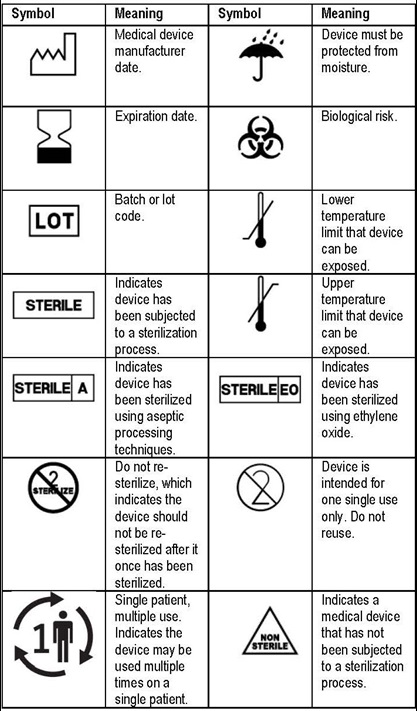
Paying attention to device labels is important to ensuring that the device can be used safely. According to the U.S. Food and Drug Administration (FDA), manufacturers of certain medical devices and products must include labeling on or with their devices. ‘Labeling’ includes all labels and other written printed or graphic information on the equipment, device or wrapper which includes but is not limited to instructions for use (IFU). Additionally, The Joint Commission requires that organizations follow the manufacturer’s written IFU to ensure the end-user understands how to use, clean, disinfect, reprocess and store medical devices.
Below are the six criteria manufacturers generally include in device labeling:
- Reflect the intended use of the device (e.g., single use, single patient use).
- Advise the end-user to thoroughly clean device.
- Indicate the correct microbiocidal process (e.g., sterilization, high-, intermediate- or low- level disinfection) for the device based on the intended use.
- Have technically feasible instructions.
- Have comprehensive instructions.
- Have understandable instructions.
In 2016, the FDA published a final rule (21 CRF Parts 660, 801 and 809) which revised its medical device labeling regulation to allow for the optional inclusion of symbols in labeling without additional explanatory text (e.g., ‘stand-alone’ symbols) if certain criteria were met.1 A device label may include important symbols which will assist the end user to easily understand key information about the item at a glance. For example, many commercially prepared sterile devices will include a manufactured date (the date the item was manufactured) which should not get confused with the expiration date (the date the item may no longer be used). Additionally, the label also may include symbols that indicate temperature and humidity requirements for storage. The table above shows examples of common symbols that may be included on packaged sterile products and their meanings. Below is an example of a label of a fictitious product showing symbols that may be included on the label.2

The hierarchical approach to infection prevention for packaged sterile supplies and devices
Another aspect to ensuring the safety of packaged sterile devices and instruments relates to monitoring temperature and humidity. The following hierarchical approach can guide your practices around storage of these products.
Rules and regulations: The first level of the hierarchy is ensuring that your organization is compliant with all building code requirements. Deemed organizations must fulfill Centers for Medicare and Medicaid Services (CMS) ventilation requirements which outline criteria for new or renovated existing facilities (constructed or plans approved on or after July 5, 2016). These are provided in the 2012 edition of NFPA 99 which references the 2008 edition of ASHRAE 170 table 7.1. If your local authority has published building codes, then your organization must meet the most restrictive requirement.
If your organization is storing sterile items in a room designated as a Central Medical and Surgical Supply Area, the following will be required, per ASHRAE Standard 170-2008:
- Positive air pressure relationship to adjacent areas
- Minimum outdoor air exchange 2 per hour
- Minimum total air exchange 4 per hour
- Maximum relative humidity 60%
- Temperature range 72 to 78 F or 22 to 26 C
CMS requirements: Depending on the type of facility, organizations must meet Conditions of Participation (CoP) or Conditions for Coverage (CfC). CMS requires that sterile packages are stored so that sterility is not compromised, and sterile items are inspected for integrity before use.
Manufacturer’s IFU: Organizations must follow the manufacturer's instructions for storage as indicated on the label. If, for example, the manufacturer of the sterile supply item requires a specific temperature and humidity requirement for storage, your organization would need to meet that requirement.
Evidence-based guidelines (EBGs) and national standards: Your organization may refer to EBGs and national standards for guidance as to how sterile supplies should be stored. Most EBGs agree that sterile supply areas must be clean, well ventilated and protect supplies from contamination, moisture, dust, temperature extremes, and humidity extremes. Whether you are storing supplies in a designated Central Medical Surgical Supply Area or in a storage room with mixed clean and sterile supplies, you should store those supplies in a manner to protect from contamination and maintain the integrity of the packaging from damage.
Safety actions to consider:
Organizations can take the following actions to ensure that their supplies and devices are stored appropriately and safely with the goal to keep patients safe from infection and other potential harm from supplies and devices that are expired or otherwise compromised.
- Educate staff to recognize the labeling used for supplies and devices, including the stand-alone symbols and their meanings.
- Provide posters and other graphic devices that are quick references to the meanings of the stand-alone symbols.
- Educate staff to follow the hierarchical approach to infection prevention for packaged sterile supplies and devices.
- Educate staff to recognize when a commercially prepared sterile medical device would be inappropriate to use (e.g., expired, torn or damaged packaging). Include next steps to stop the line and report concerns per your organization’s guidelines.
- Educate staff on where to find information specific to IFU should a question or concern be identified.
Resources:
- AT Hazlett and S Colburn. Using Symbols to Convey Information in Medical Device Labeling (web page). Updated July 19, 2018.
- U.S. Food and Drug Administration. UDI Basics (web page). Updated May 14, 2019.
Other resources:
- U.S. Food and Drug Administration. Quality System Regulation Labeling Requirements (web page). Updated Aug. 31, 2018.
- U.S. Food and Drug Administration. Guidance Document: Labeling – Regulatory Requirements for Medical Devices (FDA 89-4203). September 1989.
- The Joint Commission. Standards FAQs: Temperature and Humidity Requirements – Guidance for Storage of Sterile Supplies. Last reviewed Oct. 19, 2021.
Note: This is not an all-inclusive list.
Legal disclaimer: This material is meant as an information piece only; it is not a standard or a Sentinel Event Alert.
The intent of Quick Safety is to raise awareness and to be helpful to Joint Commission-accredited organizations.
The information in this publication is derived from actual events that occur in health care.
©The Joint Commission, Division of Healthcare Improvement


