Quick Safety 33: Improperly sterilized or HLD equipment – a growing problem
Issue:
In 2014, The Joint Commission addressed improperly sterilized or high-level disinfected (HLD) equipment in Quick Safety Issue 2. Three years later, improperly sterilized or HLD equipment continues to be a frequently scored noncompliant standard — Infection Control (IC) 02.02.01. The Joint Commission encourages leadership to carefully oversee these processes and ensure that staff is properly trained and has the resources needed to adequately perform these critical functions. This Quick Safety replaces the May 2014 issue, and includes updated and expanded safety actions to help leaders address this growing problem.
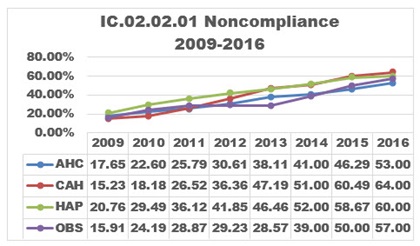
Standard IC.02.02.01 requires organizations to reduce the risk of infections associated with medical equipment, devices and supplies. This standard is applicable to Joint Commission-accredited hospitals (HAP), critical access hospitals (CAH), ambulatory (AHC) and office-based surgery (OBS) facilities. Of these, the most vulnerable locations for lapses in sterilization or HLD of equipment are ambulatory care sites (including office-based surgery facilities) and decentralized locations in hospitals, even though the data shows higher noncompliance rates for critical access hospitals and hospitals. The noncompliance rate by accreditation program for 2009-2016 is shown in the graph.
The consequences of failed processes may result in serious outcomes for the organization. These include:
- Placing patients at risk for contamination
- Causing potential outbreaks
- Potential loss of Joint Commission accreditation
- Potential loss of Centers for Medicare and Medicaid Services (CMS) deeming status
- Bad publicity, lost business and a damaged reputation
- Litigation
To this point, The Joint Commission has found that from 2013-2016, immediate threat to life (ITL) declarations directly related to improperly sterilized or HLD equipment increased significantly. In 2016, 74 percent of all ITLs were related to improperly sterilized or HLD equipment.
Breaches in equipment sterilization and HLD processes can result in outbreaks of HIV, and hepatitis B and C, as well as the transmission of bacterial infecting agents, such as Pseudomonas aeruginosa, E.coli, MRSA (methicillin resistant Staphylococcus aureaus), salmonella, and Clostridium sordellii.
In many instances, the problem is long-standing at an organization, and the true scope of the problem isn’t known until there is an outbreak, which may require assistance with local or state departments of health or the Centers for Disease Control and Prevention (CDC).
According to reports to The Joint Commission’s Office of Quality and Patient Safety, findings from non-complying organizations include:
 The mistaken belief that the risk of passing bloodborne pathogens or bacterial agents to patients is low or nonexistent
The mistaken belief that the risk of passing bloodborne pathogens or bacterial agents to patients is low or nonexistent- Staff lack the knowledge or training required to properly sterilize or HLD equipment.
- Staff don’t have access to or lack knowledge of evidence-based guidelines.
- Lack of leadership oversight.
- Sterilization or HLD of equipment becomes a low priority within the organization.
- Lack of a culture of safety that supports the reporting of safety risks.
- Processes for sterilization or HLD are not followed (i.e., staff take shortcuts).
- The time frames for proper sterilization or HLD of equipment are not followed.
- There is no dedicated staff person to oversee the proper sterilization or HLD of equipment.
- Facility design or space issues prevent proper sterilization or HLD of equipment (e.g., processing takes place in a small room that also is used for storage).
- Lack of monitoring or documentation of sterilization or HLD of equipment, which makes it difficult to track the use of equipment on a specific patient, complicating the patient notification process when an outbreak occurs.
- Equipment is spread throughout the facility and may be processed or stored in numerous locations, making it difficult to track the equipment for documentation purposes.
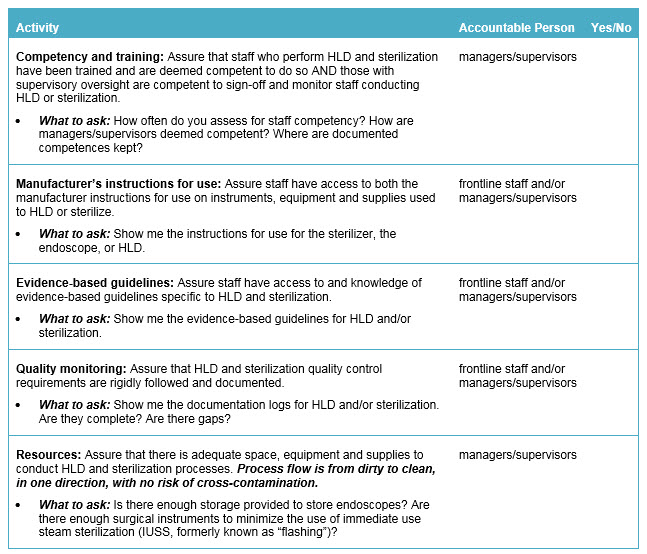
Safety Actions to Consider:
The following is a checklist for leadership to follow to address sterilization and HLD in order to help protect patients from the transmission of disease and bacterial agents:
 |
Resources:
- American National Standards Institute (ANSI) and Association for the Advancement of Medical Instrumentation (AAMI): Comprehensive guide to steam sterilization and sterility assurance in health care facilities, ANSI/AAMI ST79:2010 & A1:2010 &A2:2011 & A3:2012
- American National Standards Institute (ANSI) and Association for the Advancement of Medical Instrumentation (AAMI): Chemical sterilization and high-level disinfection in health care facilities, ANSI/AAMI ST58:2013
- Centers for Disease Control and Prevention: CDC Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008
- Association of periOperative Registered Nurses: AORN 2016 Perioperative Standards and Recommended Practices for Sterilization
- The Joint Commission: High-Level Disinfection (HLD) and Sterilization BoosterPak.
Note: This is not an all-inclusive list.
Legal disclaimer: This material is meant as an information piece only; it is not a standard or a Sentinel Event Alert. The intent of Quick Safety is to raise awareness and to be helpful to Joint Commission-accredited organizations. The information in this publication is derived from actual events that occur in health care.
©The Joint Commission, Division of Health Care Improvement


